DISCOVERY OF THE ATOMIC NUCLEUS
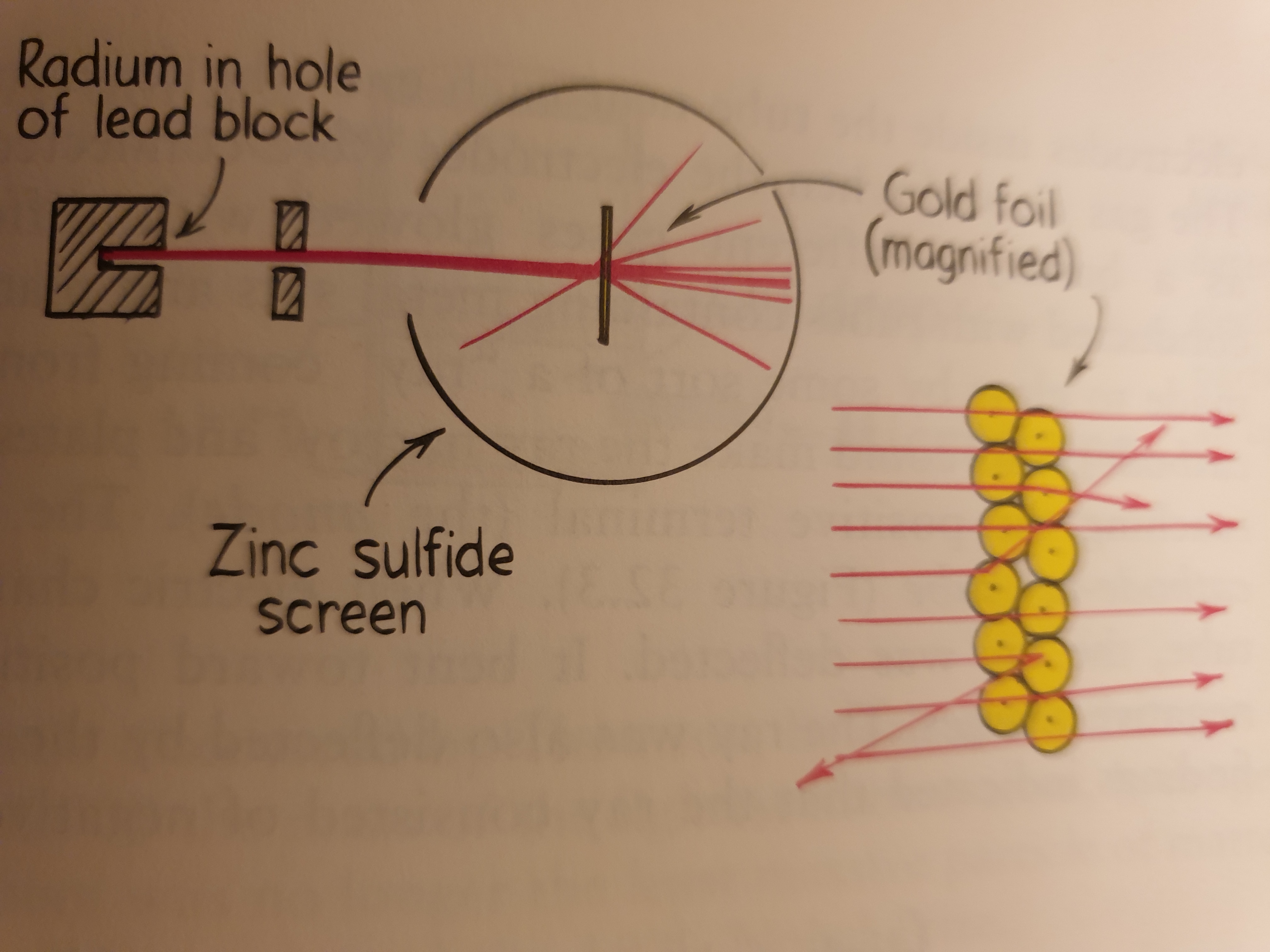
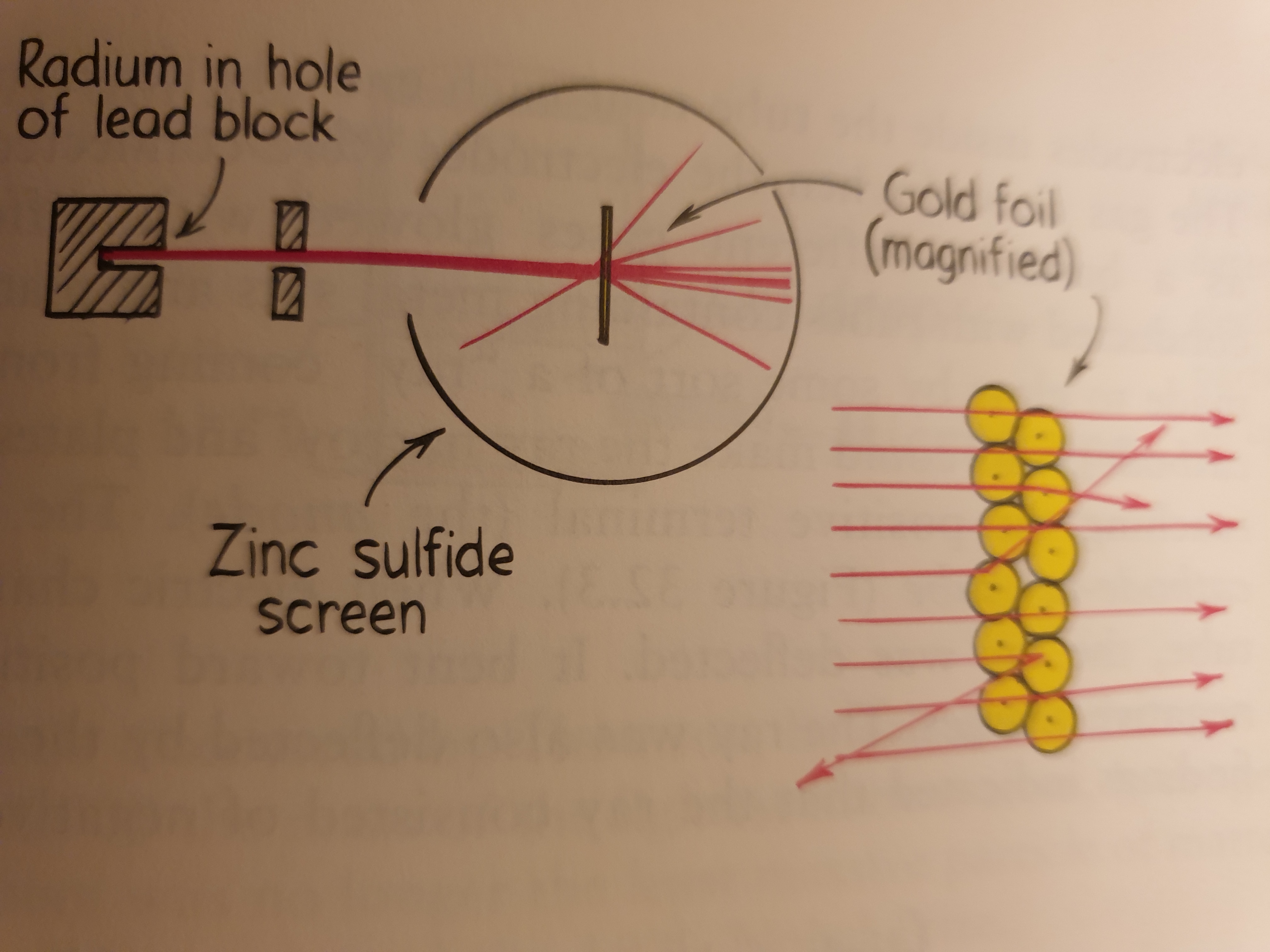
Half a dozen years after Einstein announced the photoelectric effect, the New-Zealand born British physicist Ernest Rutherford oversaw his now famous gold-foil experiment. This significant experiment showed that the atom is mostly empty space, with most of its mass concentrated in the central region: the atomic nucleus.
In Rutherford experiment, a beam of positively charged particles (alpha particles) from a radioactive source was directed through a sheet of extremely thin gold foil. Because alpha particles are thousands of times more massive than electrons, it was expected that the stream of alpha particles would not be impeded as it passed through the “atomic pudding”. This was indeed observed for the most part. Nearly all alpha particles passed through the gold foil with little or no deflection and produced a spot of light when they hit a fluorescent screen beyond the foil, but some particles were deflected from their straight-line paths as they emerged. A few alpha particles were widely deflected, and a small number were even scattered backward! These alpha particles must have hit something relatively massive (but what?). Rutherford reasoned that the undeflected particles traveled through regions of the gold that were empty space, while the small number of deflected particles were repelled from extremely dense, positively charged central cores. Each atom, he concluded, must contain one of these cores, which he named the atomic nucleus.
REVIEW QUESTIONS: (can easily be answered from the text)
1- Why do most alpha particles fired through a piece of gold foil emerge almost undeflected?
2- Why do a few alpha particles fired at a piece of gold foil bounce backward?
AMAZING PHYSICS (Nuclear Physics)
QUESTION:
When a U-235 nucleus absorbs a neutron and undergoes a nuclear fission, about 200 MeV of energy is released. But in what form? Interestingly, most of this energy initially appears in the form of:
a) gamma rays
b) kinetic energy of the emitted neutrons
c) kinetic energy of the fission fragments
d) heat
e) each of these, about equally
ANSWER: (c)
Some energy is emitted in the form of gamma rays and some goes into the kinetic energy of the emitted neutrons, but most of the energy of nuclear fission is in the kinetic energy of the fission fragments. The positively-charged fragments repel each other and fly apart at high speed. Soon, their energy is shared among many atoms as internal energy. It then spreads as heat.
- Enseignant: CHAMS_EDDINE KHIARI (Enseignant)