X-RAYS AND RADIOACTIVITY
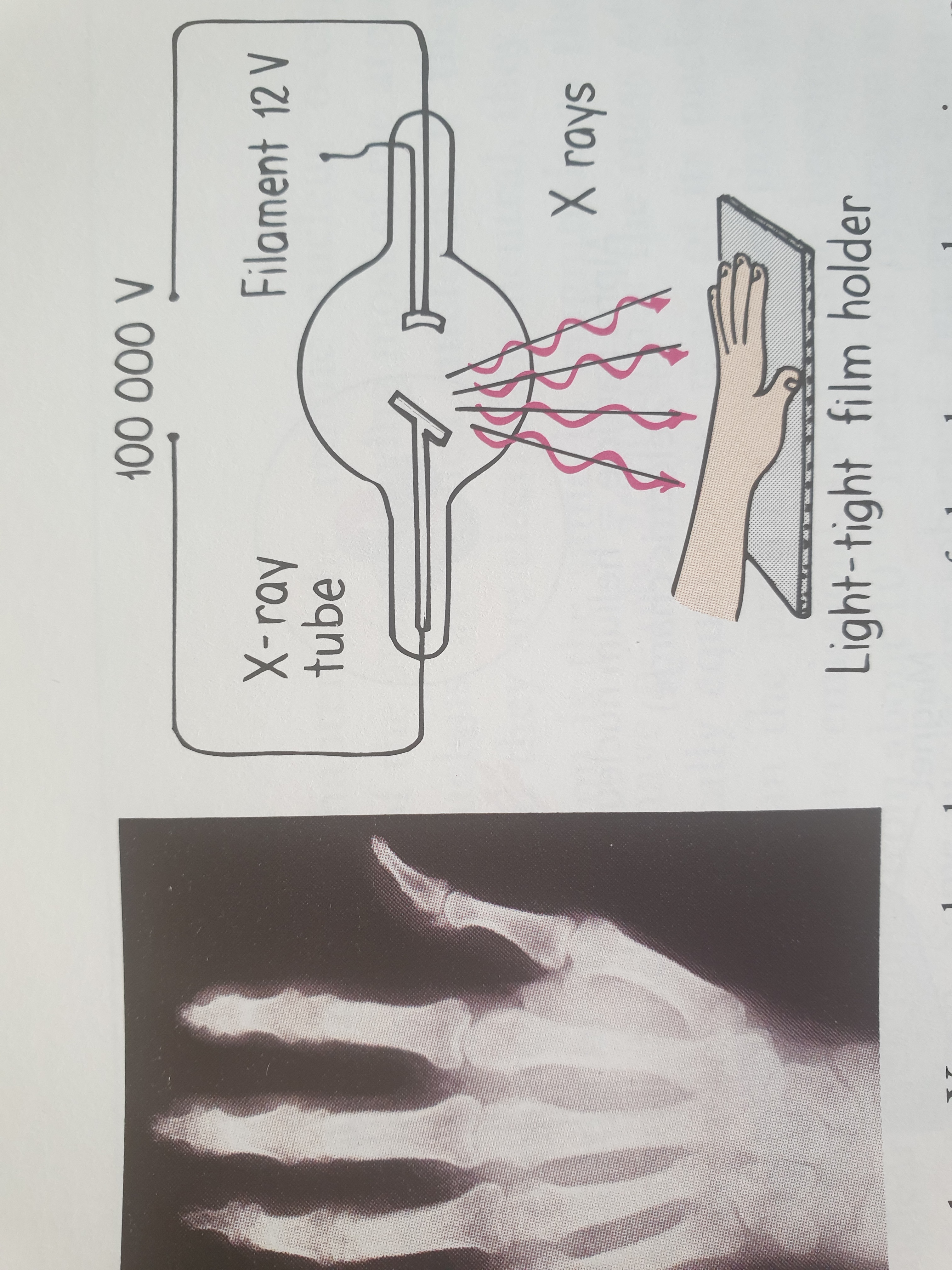
Before the turn of the twentieth century, the German physicist Wilhelm Roentgen discovered a “new kind of ray” produced by a beam of “cathode rays” (later found to be electrons) striking the glass surface of a gas-discharge tube. He named these X-rays (rays of an unknown nature). Roentgen found that X-rays could pass through solid materials, could ionize the air, showed no refraction in glass, and were undeflected by magnetic fields. Today, we know that x-rays are high-frequency electromagnetic fields, usually emitted by the de-excitation of the innermost orbital electrons of atoms. Whereas the electron current in a fluorescent lamp excites the outer electrons of atoms and produces ultraviolet and visible photons, a more energetic beam of electrons striking a solid surface excites the innermost electrons and produces higher-frequency photons of X-radiation.
X-ray photons have high energy and can penetrate many layers of atoms before being absorbed or scattered. X-rays do this when they pass through your soft tissues to produce images of the bones inside of your body (see figure below). In a modern X-ray tube, the target of the electron beam is a metal plate rather than the glass wall of the tube.
Two months after Roentgen announced his discovery of X-rays, the French physicist Antoine Henri Becquerel tried to find out whether any elements spontaneously emitted X-rays. To do this, he wrapped a photographic plate in black paper to keep out the light and put pieces of various elements against the wrapped plate. From Roentgen’s work, Becquerel knew that, if these materials emitted X-rays, the rays would go through the paper and blacken the plate. He found that, although most elements produced no effect, uranium did produce rays. It was soon discovered that similar rays are produced by other elements such as, thorium, actinium, and two new elements discovered by Marie and Pierre Curie: polonium and radium. The emission of these rays was evidence of much more drastic changes in the atom than atomic excitation. These rays were the result of not changes in the electron energy states of the atom but of changes occurring within the central atomic core: the nucleus. These rays were the result of of a spontaneous chipping apart of the atomic nucleus: radioactivity.
REVIEW QUESTIONS (can easily be answered from the text):
1- What did the physicist Roentgen discover about a cathode-ray beam striking a glass surface?
2- What is the similarity between a beam of X-rays a beam of light? What is the principal difference between the two?
3- What did the physicist Becquerel discover about uranium?
4- What two elements did Pierre and Marie Curie discover?
AMAZING PHYSICS (Nuclear Physics)
QUESTION:
Compared to Hydrogen , the element Helium has:
a) more mass and is larger in size.
b) more mass and is about the same in size.
c) more mass and is smaller in size.
d) none of the above.
ANSWER: c
The nucleus of helium has four nucleons (two protons and two neutrons) compared to hydrogen’s one (one proton). So, it is about four times as massive as hydrogen. The nucleus of helium has twice the electric charge of hydrogen and pulls its electrons into a tighter orbit than hydrogen. Helium is a smaller but heavier atom then hydrogen.
- Enseignant: CHAMS_EDDINE KHIARI (Enseignant)