THE ELEMENTS
When a substance is composed of atoms of the same kind, we call that substance an element. A pure 24-carat gold ring, for example, is composed only of gold atoms. A gold ring with a lower carat value is composed of gold and other elements, such as nickel. The silvery liquid in a thermometer is the element mercury. The entire liquid consists of only mercury atoms. Of course, if a substance contains only a single kind of atom, it can correctly be called an element. An atom of a particular element is the smallest sample of that element. Although atom and element are used interchangeably, element is preferred when referring to macroscopic quantities. For example, we speak of isolating a mercury atom from a flask of the element mercury.
The lightest element of all is hydrogen. In the universe at large, it is the most abundant element. More than 90% of the atoms in the known universe are hydrogen atoms. Helium, the second-lightest elements, provides most of the remaining atoms in the universe. Heavier atoms in our surroundings were manufactured by the fusion of the light elements in the hot, high pressure cauldrons deep within in the interiors of stars. The heaviest elements form when huge stars implode and then explode (supernovas). Nearly all the elements on Earth are remnants of stars that exploded long before the solar system came into existence.
To date, 118 elements are known. Of these, 94 occur in nature. The others are produced in laboratory with high-energy atomic accelerators and nuclear reactors. The laboratory-produced elements are too unstable (radioactive) to occur naturally in appreciable quantities.
From a pantry containing less than 100 elements, we have the atoms that constitute almost every simple, complex, living or non-living substance in the known universe. More than of the material on Earth is formed from only about a dozen of the elements. The other elements are relatively rare. Living things are composed primarily of five elements: oxygen (O), carbon (C), hydrogen (H), nitrogen (N) and calcium (Ca). The letters in parentheses represent the chemical symbols for these elements.
REVIEW QUESTIONS: (can easily be answered from the text)
1- What is the lightest of the elements?
2- What is the most abundant element in the universe?
3- How were elements heavier than hydrogen formed?
4- Where did the heaviest elements originate?
5- What are the five most common elements in things?
AMAZING PHYSICS (Linear Motion)
QUESTION:
A motorist wishes to travel 40 kilometers at an average speed of 40 kilometers per hour (40 km/h). During the first 20 kilometers, an average speed of 40 km/h is maintained. During the next 10 kilometers, however, the motorist goofs off and averages only 20 km/h. To drive the last 10 kilometers and average 40 km/h, the motorist must drive:
a) 60 km/h
b) 80 km/h
c) 90 km/h
d) faster than the speed of light
ANSWER: d (faster than the speed of light!)
EXPLANATION:
He would have to travel at an infinite speed and finish the last 10 kilometers in zero time and attain an average speed of 40 km/h!
Why? Because he has an hour to make the trip and his one hour is up (i.e. it is over) at the 30-km point. He spent ½ hour to the halfway point, 20 kilometers, and another ½ hour when he averaged 20km/h over that 10-kilometer stretch. So, he would have to cover the entire 40 kilometers in 1 hour. This means, the last 10 kilometers in no time at all!
- Teacher: CHAMS_EDDINE KHIARI (Enseignant)
X-RAYS AND RADIOACTIVITY
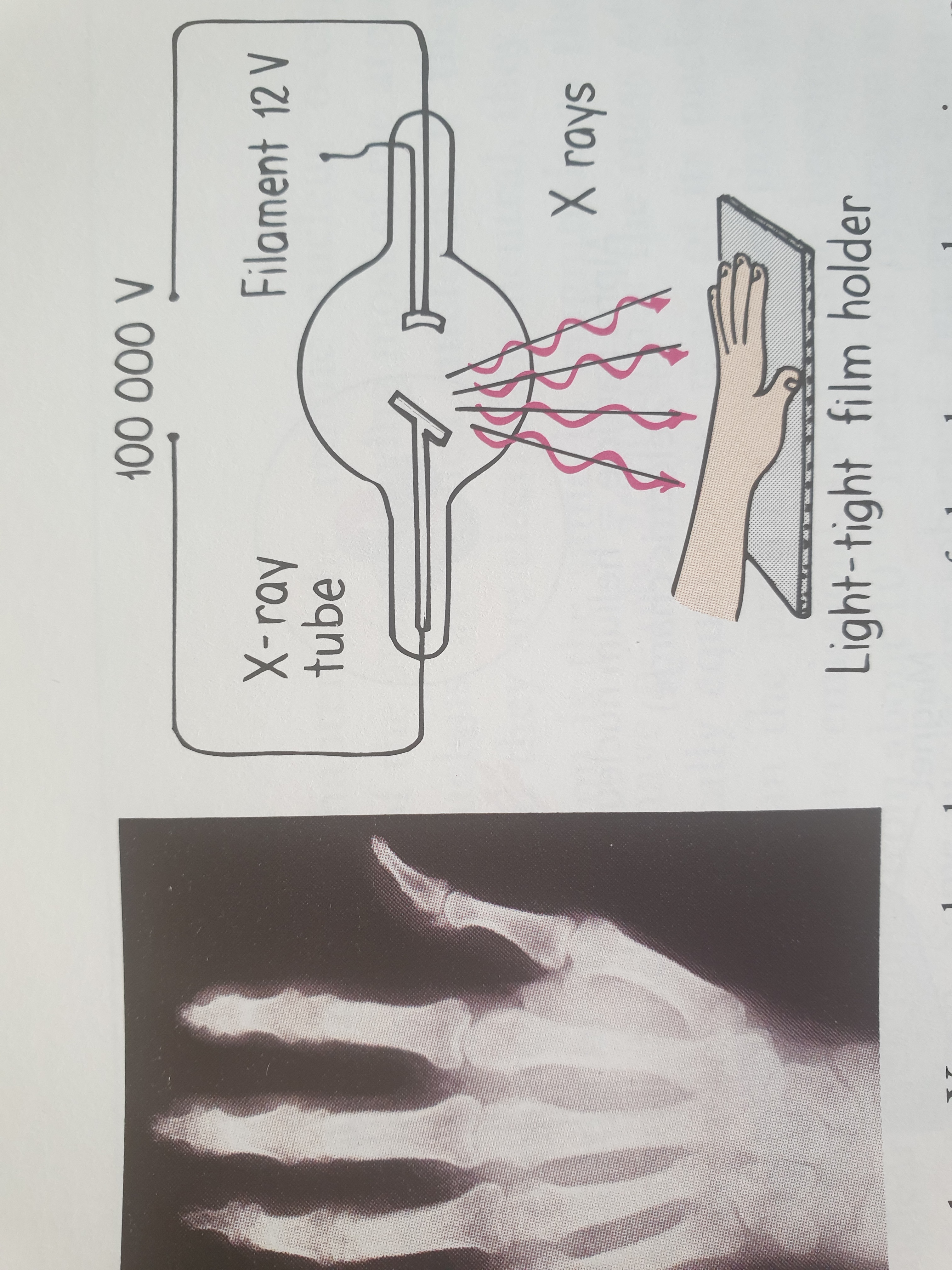
Before the turn of the twentieth century, the German physicist Wilhelm Roentgen discovered a “new kind of ray” produced by a beam of “cathode rays” (later found to be electrons) striking the glass surface of a gas-discharge tube. He named these X-rays (rays of an unknown nature). Roentgen found that X-rays could pass through solid materials, could ionize the air, showed no refraction in glass, and were undeflected by magnetic fields. Today, we know that x-rays are high-frequency electromagnetic fields, usually emitted by the de-excitation of the innermost orbital electrons of atoms. Whereas the electron current in a fluorescent lamp excites the outer electrons of atoms and produces ultraviolet and visible photons, a more energetic beam of electrons striking a solid surface excites the innermost electrons and produces higher-frequency photons of X-radiation.
X-ray photons have high energy and can penetrate many layers of atoms before being absorbed or scattered. X-rays do this when they pass through your soft tissues to produce images of the bones inside of your body (see figure below). In a modern X-ray tube, the target of the electron beam is a metal plate rather than the glass wall of the tube.
Two months after Roentgen announced his discovery of X-rays, the French physicist Antoine Henri Becquerel tried to find out whether any elements spontaneously emitted X-rays. To do this, he wrapped a photographic plate in black paper to keep out the light and put pieces of various elements against the wrapped plate. From Roentgen’s work, Becquerel knew that, if these materials emitted X-rays, the rays would go through the paper and blacken the plate. He found that, although most elements produced no effect, uranium did produce rays. It was soon discovered that similar rays are produced by other elements such as, thorium, actinium, and two new elements discovered by Marie and Pierre Curie: polonium and radium. The emission of these rays was evidence of much more drastic changes in the atom than atomic excitation. These rays were the result of not changes in the electron energy states of the atom but of changes occurring within the central atomic core: the nucleus. These rays were the result of of a spontaneous chipping apart of the atomic nucleus: radioactivity.
REVIEW QUESTIONS (can easily be answered from the text):
1- What did the physicist Roentgen discover about a cathode-ray beam striking a glass surface?
2- What is the similarity between a beam of X-rays a beam of light? What is the principal difference between the two?
3- What did the physicist Becquerel discover about uranium?
4- What two elements did Pierre and Marie Curie discover?
AMAZING PHYSICS (Nuclear Physics)
QUESTION:
Compared to Hydrogen , the element Helium has:
a) more mass and is larger in size.
b) more mass and is about the same in size.
c) more mass and is smaller in size.
d) none of the above.
ANSWER: c
The nucleus of helium has four nucleons (two protons and two neutrons) compared to hydrogen’s one (one proton). So, it is about four times as massive as hydrogen. The nucleus of helium has twice the electric charge of hydrogen and pulls its electrons into a tighter orbit than hydrogen. Helium is a smaller but heavier atom then hydrogen.
- Teacher: CHAMS_EDDINE KHIARI (Enseignant)
DISCOVERY OF THE ATOMIC NUCLEUS
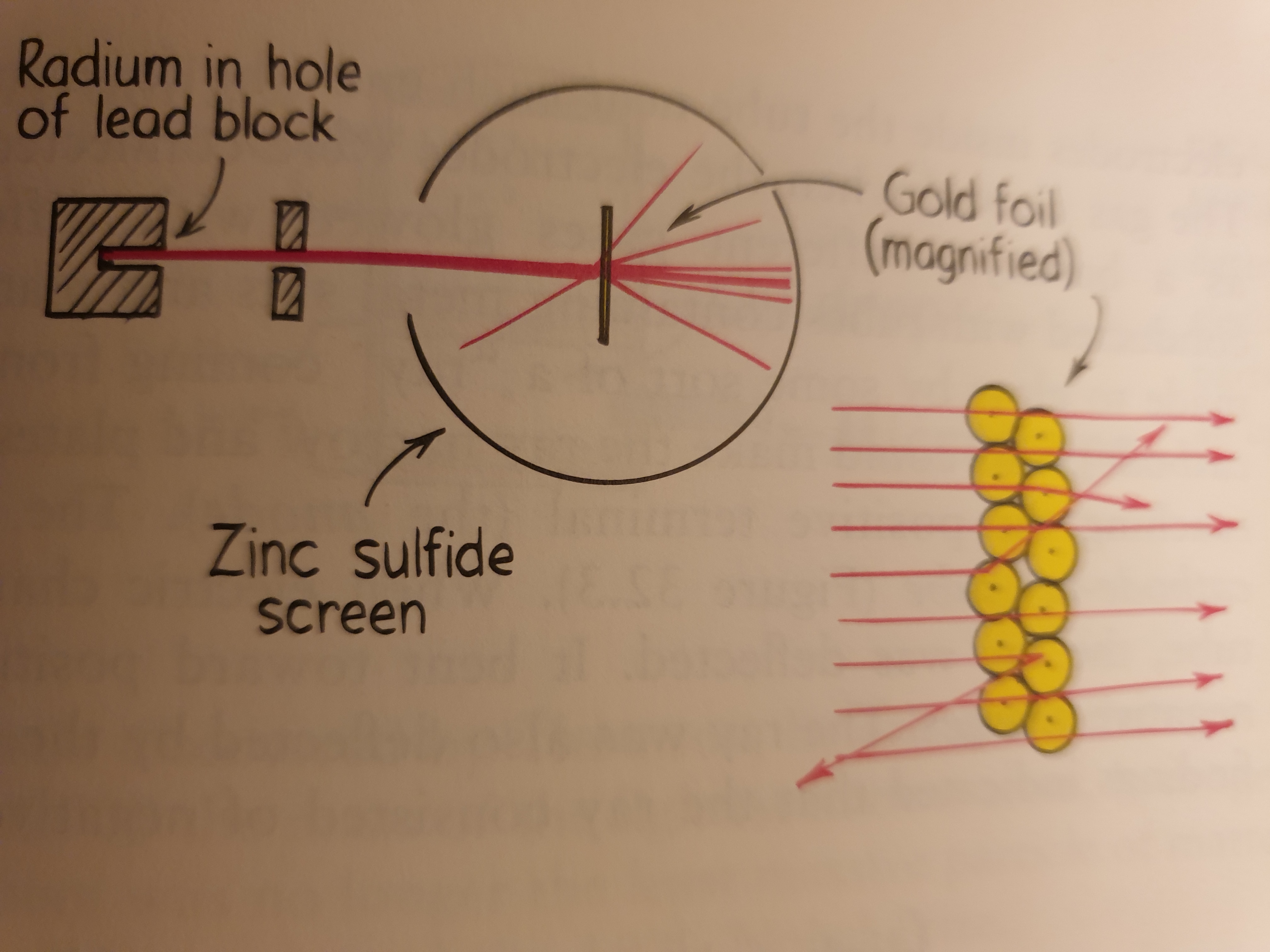
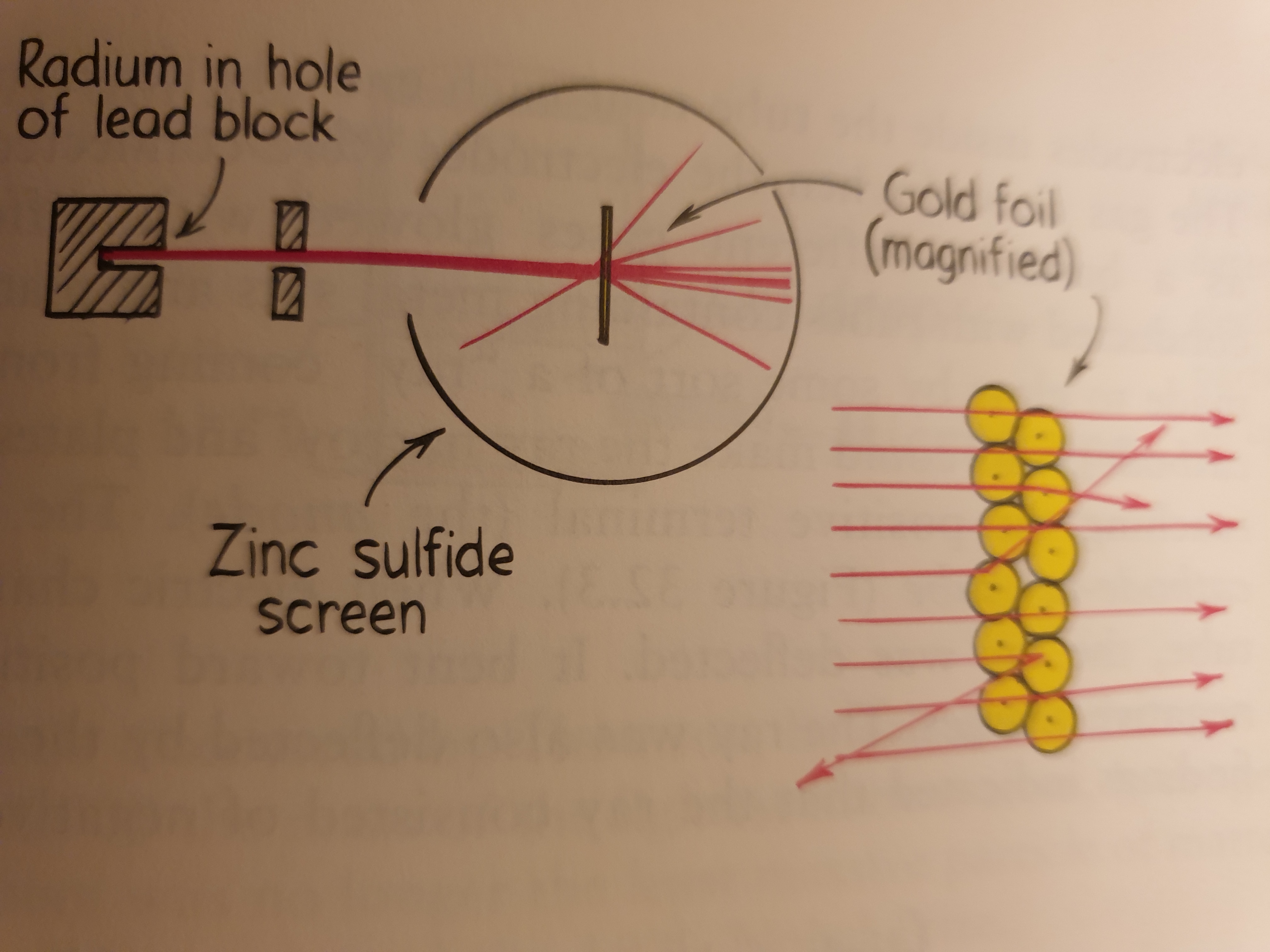
Half a dozen years after Einstein announced the photoelectric effect, the New-Zealand born British physicist Ernest Rutherford oversaw his now famous gold-foil experiment. This significant experiment showed that the atom is mostly empty space, with most of its mass concentrated in the central region: the atomic nucleus.
In Rutherford experiment, a beam of positively charged particles (alpha particles) from a radioactive source was directed through a sheet of extremely thin gold foil. Because alpha particles are thousands of times more massive than electrons, it was expected that the stream of alpha particles would not be impeded as it passed through the “atomic pudding”. This was indeed observed for the most part. Nearly all alpha particles passed through the gold foil with little or no deflection and produced a spot of light when they hit a fluorescent screen beyond the foil, but some particles were deflected from their straight-line paths as they emerged. A few alpha particles were widely deflected, and a small number were even scattered backward! These alpha particles must have hit something relatively massive (but what?). Rutherford reasoned that the undeflected particles traveled through regions of the gold that were empty space, while the small number of deflected particles were repelled from extremely dense, positively charged central cores. Each atom, he concluded, must contain one of these cores, which he named the atomic nucleus.
REVIEW QUESTIONS: (can easily be answered from the text)
1- Why do most alpha particles fired through a piece of gold foil emerge almost undeflected?
2- Why do a few alpha particles fired at a piece of gold foil bounce backward?
AMAZING PHYSICS (Nuclear Physics)
QUESTION:
When a U-235 nucleus absorbs a neutron and undergoes a nuclear fission, about 200 MeV of energy is released. But in what form? Interestingly, most of this energy initially appears in the form of:
a) gamma rays
b) kinetic energy of the emitted neutrons
c) kinetic energy of the fission fragments
d) heat
e) each of these, about equally
ANSWER: (c)
Some energy is emitted in the form of gamma rays and some goes into the kinetic energy of the emitted neutrons, but most of the energy of nuclear fission is in the kinetic energy of the fission fragments. The positively-charged fragments repel each other and fly apart at high speed. Soon, their energy is shared among many atoms as internal energy. It then spreads as heat.
- Teacher: CHAMS_EDDINE KHIARI (Enseignant)
PRESSURE
Gases are made up of atoms and molecules, which are in constant and rapid motion. The atoms and molecules are constantly hitting the walls and the gas container. In doing so, they exert pressure on the walls.
Gases have no definite shape or volume. The shape and the volume of a gas depend on its container. Compared with solids and liquids, the molecules of a gas are relatively far apart. Hence, they can be compressed, or forced into a smaller space. But if the volume of a gas is decreased, its pressure is increased, because the molecules hit the walls more often and more rapidly. Thus, pressure is in inverse proportion to volume.
A liquid, on the other hand, cannot be compressed. If we try to force a liquid into a smaller space, it seeks a way out of the container. the pressure which a liquid exerts on the walls of a container is equal in all directions. This is why liquids are used for transmitting power in different directions, in hydraulic brakes and elevators.
The more a gas is compressed, the greater its resistance to compression. If a large amount of gas is forced into a small space, it becomes difficult to compress further. Under very high pressure, compressed gas can be used for transmitting power.
As gas pressure is increased, the molecules are forced closer together. If this continues, the molecules eventually become attached to one another. At this point, the gas changes into a liquid.
The pressure of a gas changes with temperature. Pressure is in direct proportion to absolute temperature, since, the higher the temperature, the more rapid the motion of the molecules, and, consequently, the greater the pressure exerted on the walls of the container. Conversely, the higher the pressure of a gas, the higher its temperature. When a gas is compressed, it becomes hotter.
.....................................................................................................................................................................
Say whether these relationships are in direct or inverse proportion. Then, put them in the order in which they occur in the text:
a) compression of a gas: resistance to further compression.
b) change in volume: change in pressure.
c) compression of a gas: increase in temperature.
d) distance between molecules: compressibility of substances.
e) change in temperature: change in pressure.
......................................................................................................................................................................
AMAZING PHYSICS ( Newton's 3rd Law)
QUESTION: Consider an apple at rest on a table. If we call the gravitational force exerted on the apple "action", what is the "reaction force" according to Newton's third law?
ANSWER: The reaction force is the apple gravitationally pulling on Earth (and NOT the support force by the table). To identify a pair of action-reaction forces in any situation, first identify the pair of interacting objects involved. Something is interacting with something else. In this case, the whole Earth is interacting (gravitationally) with the apple. So, Earth pulls downward on the apple (call it action), while the apple pulls upward on Earth (reaction). Simply put, Earth pulls on the apple (action), the apple pulls on Earth (reaction). Better put, the is a single interaction between the apple and Earth, and they simultaneously pull on each other, with the same amount of force.
- Teacher: CHAMS_EDDINE KHIARI (Enseignant)
STANDARDS OF MEASUREMENT
In early times, measurements were made by comparing things with parts of the human body. Early units of measurement included the distance from the elbow to the fingers, the width of the hand and the width of the fingers.
Some of these human measurements are still used. For example, the inch is based on the length of half the thumb. A foot was originally the length of a man's foot. A mile was one thousand walking steps.
These units were only approximate, because their standard -the human body- was not constant. Governments tried to standardize them by using rods of fixed lengths. But these rods still varied from country to country.
During the french revolution, scientists looked for a standard of measurement which did not change. They chose the distance from the Equator to the North Pole, which is one quarter of the circumference of the Earth. One ten-millionth of this was called one meter and became the basic unit of the metric system. Other metric units are based on it.. For example, the centimeter is one hundredth of a meter.
A standard meter is marked on a platinum bar. The accuracy of measuring instruments was checked by comparing them with this bar. Nowadays, the meter is standardized by comparing it with another constant: the wavelength of a certain kind of light.
Now complete these sentences:
1- .................. included the distance from elbow to fingers, width of hand, width of finger.
2- .................. include the inch, the foot, the mile.
3- These .............. measurements were not ............ because ............... were used to standardize them, but they also varied.
4- .............. was chosen as the basic unit of ................ . Its length is ................ of ............ the Earth circumference. Other metric units are .................... .
5- The standard meter is marked on .................. . Nowadays, another constant is used: .................... .
AMAZING PHYSICS (Electrostatics)
Two oppositely charged particles, an alpha particle with 2 positive charges and a less-massive electron with a single negative charge, are attracted to each other.
1- Compared to the force that the alpha particle exerts on the electron, the electron exerts a force on the alpha particle that is:
a) greater b) the same c) smaller
2- The particle with the most acceleration is the:
d) alpha particle e) electron f) same for each
3- As the particles get closer to each other, each experiences an increase in:
g) force h) speed i) acceleration
j) all of the above k) none of the above
ANSWERS:
b) the same
e) the electron
j) all of the above
EXPLANATION:
a) By Newtons's 3rd law, the particles pull each other with equal and opposite forces.
b) By Newton's 2nd law, for the same force, the particle with less mass undergoes more acceleration.
c) By Coulomb's law, as the separation distance is decreased, the force increases. By Newton's 2nd law, as the force increases, the acceleration increases. Since the particles accelerate toward each other, their speeds increase also.
- Teacher: CHAMS_EDDINE KHIARI (Enseignant)